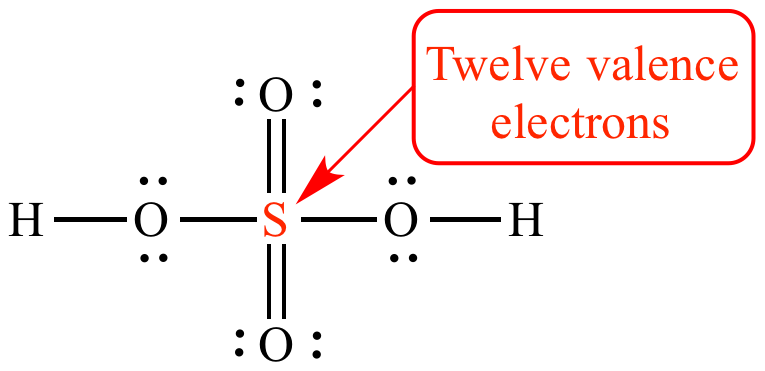
Sulfur phosphorus silicon and chlorine are common examples of elements that form an expanded octet. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom.
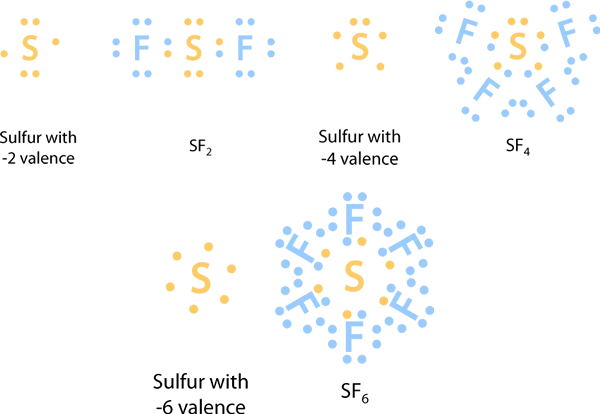
Exceptions Of The Octet Rule Study Guide Inspirit
Fluorine is more electronegative than sulfur but hydrogen is not.
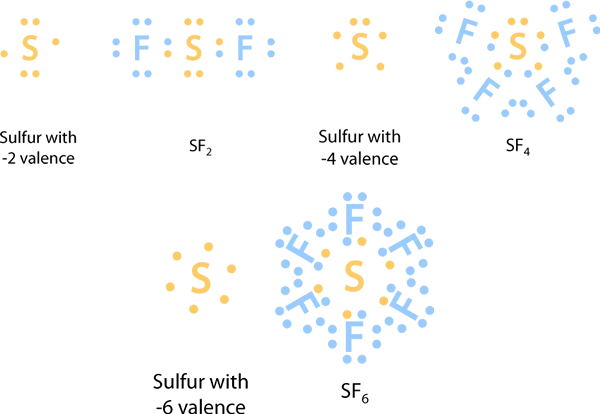
. For example is a stable compound but is not. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. As described in this answer this delocalization in a neutral molecule comes with occupying ligand-based orbitals that are roughly nonbonding with a large central atom but antibonding with a.
Notice that expanded octets only occur in compounds in which the central atom has low electronegativity relative to the ligands. Sulfur may be in the same group as Oxygen but it is in a period below Oxygen. March 22 2022 By.
Phosphorus pentachloride PCl 5 and sulfur hexafluoride SF 6 are examples of molecules that deviate from the octet rule by having more than 8 electrons around the central atom. So in addition to being octet sulfur can expand octet to have 10 or 12 electrons. Why does Sulfur accommodate expanded octet.
The octet rule can be expanded by some elements by utilizing the d-orbitals found in the third principal energy level and beyond. March 8 2022. Instead sulfur in compounds such as ceSF6 delocalizes the bonds formed with the usual octet over multiple linkages.
This extra period correlates to an extra energy level. Sulfur and phosphorus are common examples of this behavior. Neither oxygen nor sulfur expands its octet.
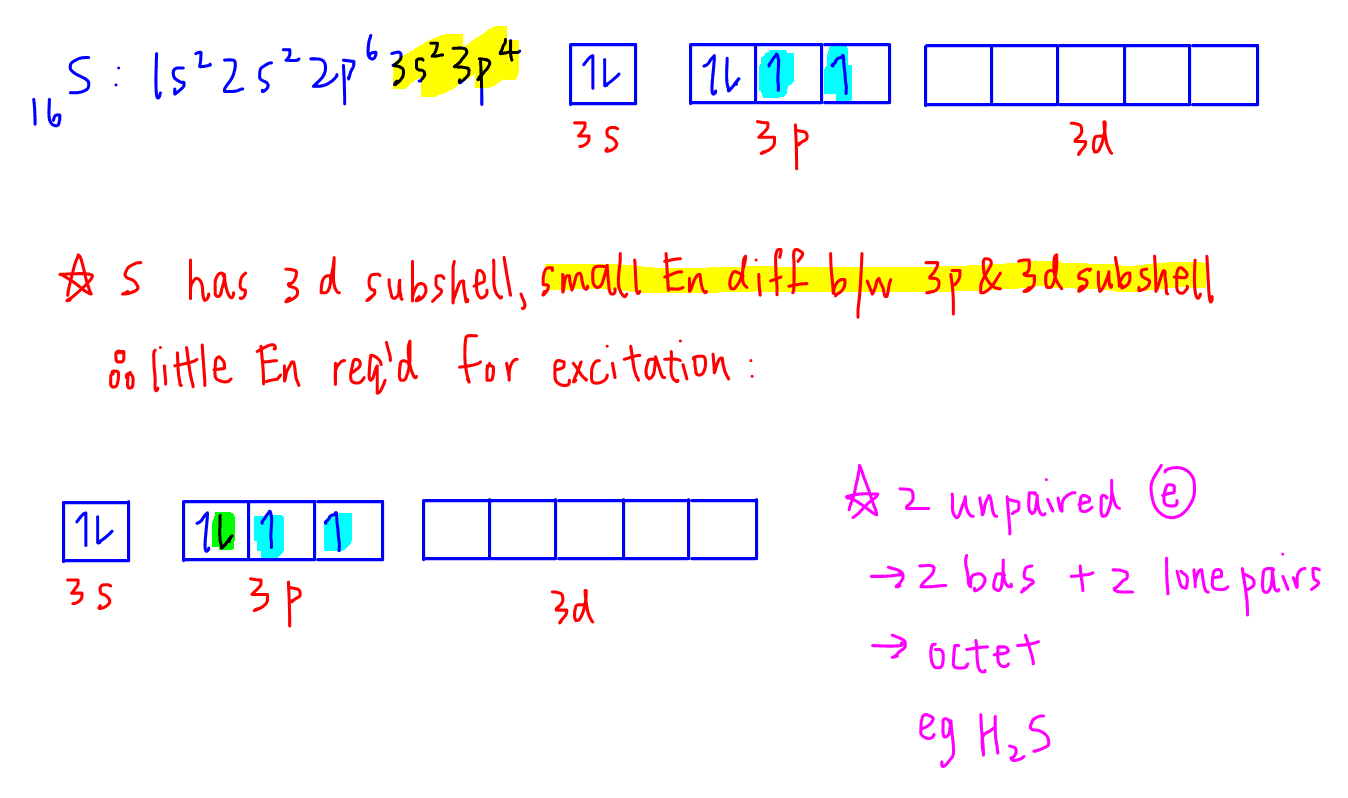
Some of the elements of the third-period and periods below can have expanded octet because they have d- sub-level. The driving force for excitation is the unpairing of electrons will allow the element to form more covalent bonds hence more energy is released from the formation of more bonds and the final configuration will be more stable. Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons.
Atoms in these periods may follow the octet rule but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Phosphorus pentachloride PCl 5 and sulfur hexafluoride SF 6 are examples of molecules that deviate from the octet rule by having more. Sulfur is in the 3rd period meaning it has a highest energy level of n3.
In my search to understand the bonding in structures like ceSF6 I found many sources that said it was because sulfur has d orbitals to accommodate an expanded octet which made sense to me. So in addition to being octet sulfur can expand octet to have 10 or 12 electrons. Each atom is surrounded by eight electrons.
Because lodine and chlorine. But I also found sources like the paper by Reed and Weinhold 1986 that say d orbitals contribute very little to the bonding in ceSF6. Sulfur can follow the octet rule as in the molecule SF 2.
Sulfur and phosphorous are in the 3d or lower periods. Sulfur phosphorus silicon and chlorine are common examples of elements that form an expanded octet. We have 2 possible answers.
There are a total of 48 valence electrons in the Lewis structure for SF6. Because sulfur is far less electronegative than oxygen.
Illustrated Glossary Of Organic Chemistry Expanded Octet Hypervalent
Why Is Sulfur So Bad At Forming Double Bonds In General Quora

0 Comments